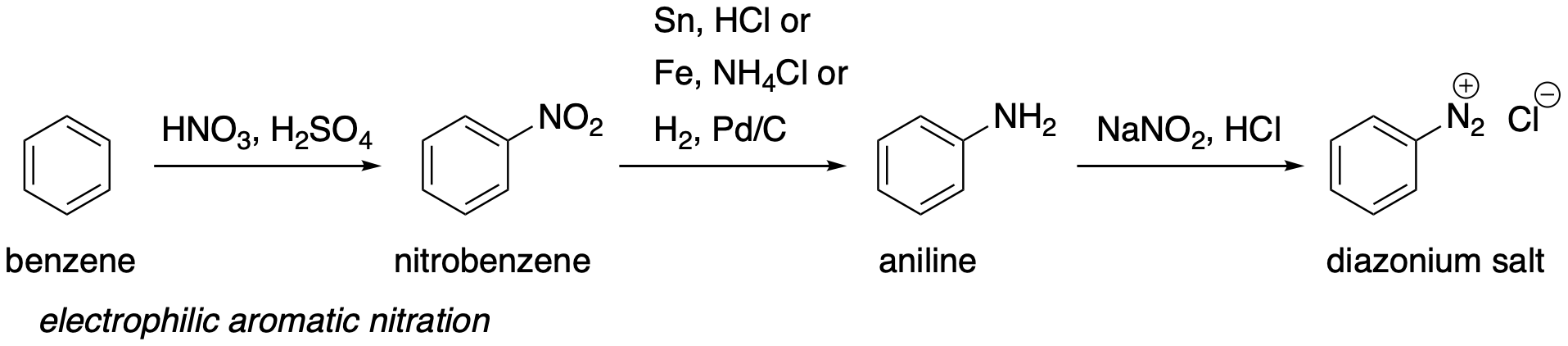
Sandmeyer Reactions
Sandmeyer reactions are an incredibly powerful set of transformations that can convert a C-N bond in an aromatic molecule to a variety of carbon-heteroatom, carbon-carbon, and carbon-H bonds.
To make aryl diazonium salts:
Start from a nitrobenzene and reduce the nitro group to an aniline with hydrogen and palladium on carbon or Sn/HCl or Fe/ammonium chloride.
Diazotize the aniline with sodium nitrite and acid (forms nitrous acid in situ)
You can take an aryl diazonium salt and convert it to many different things!